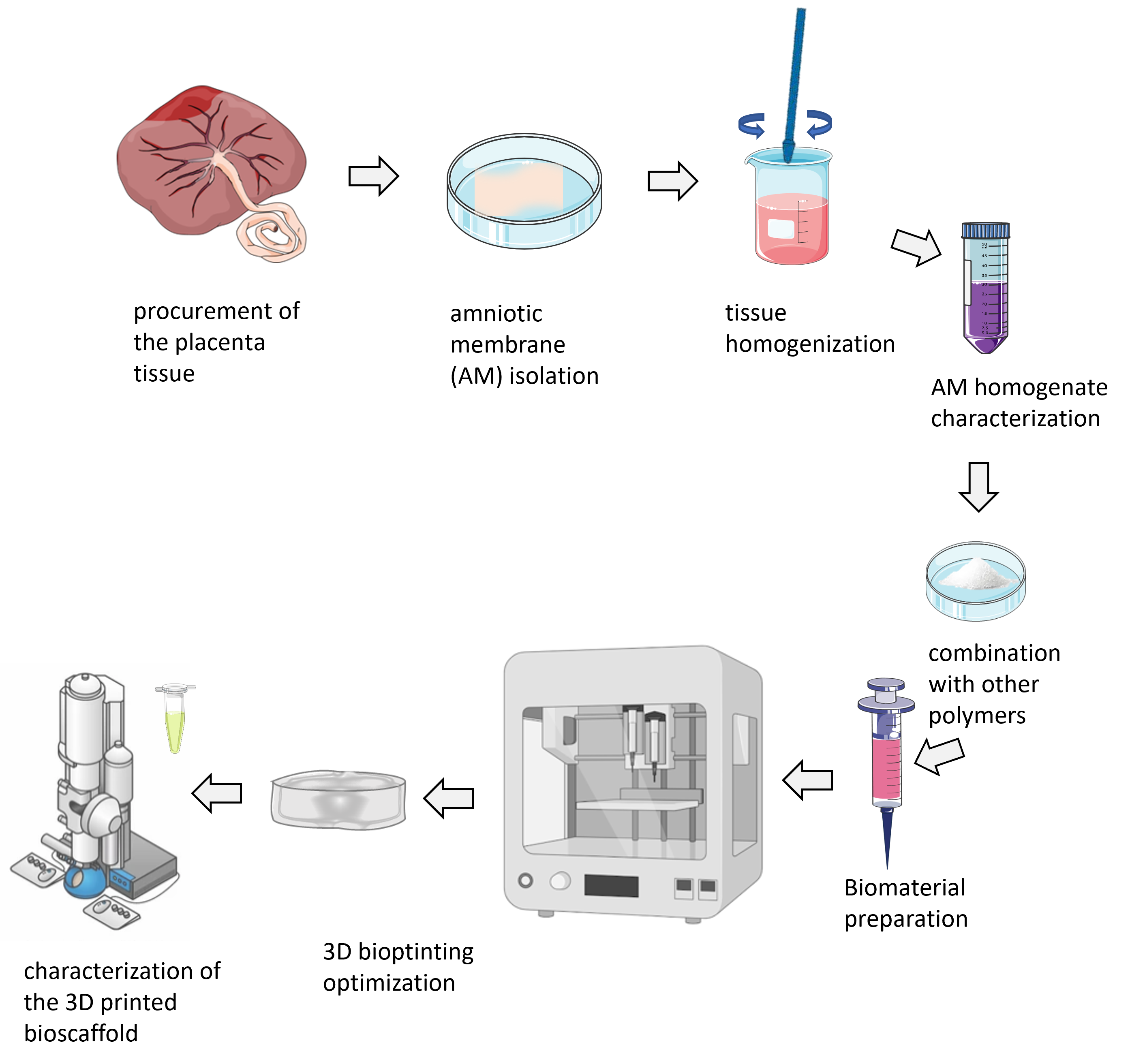
WORK PACKAGE 1
The use of 3D bioprinters in science and biotechnology is growing, especially due to the need to produce 3D tissue-mimicking systems to replace the use of laboratory animals. When it comes to the production of bioscaffolds, the advantage of 3D bioprinting is reflected in precise and customized design, simultaneous application of multiple materials, reproducibility and time efficiency. By using simple CAD (computer-aided design) software, it is possible to perform patient-specific 3D modelling or tissue modelling of the desired shape, size and porosity. Within this work package, bioscaffolds of certain physical characteristics (size, porosity, stiffness, etc.) will be printed using the extrusion method so that they correspond to the physical characteristics of the breast tissue. Homogenate from the amniotic membrane (hAM) of the human placenta in combination with natural biopolymers (eg alginate, silk fibroin) will be used as starting material. By optimizing the obtained biomaterial, we strive to develop an implant that will have an extended time of biodegradation in order to exhibit its effects.
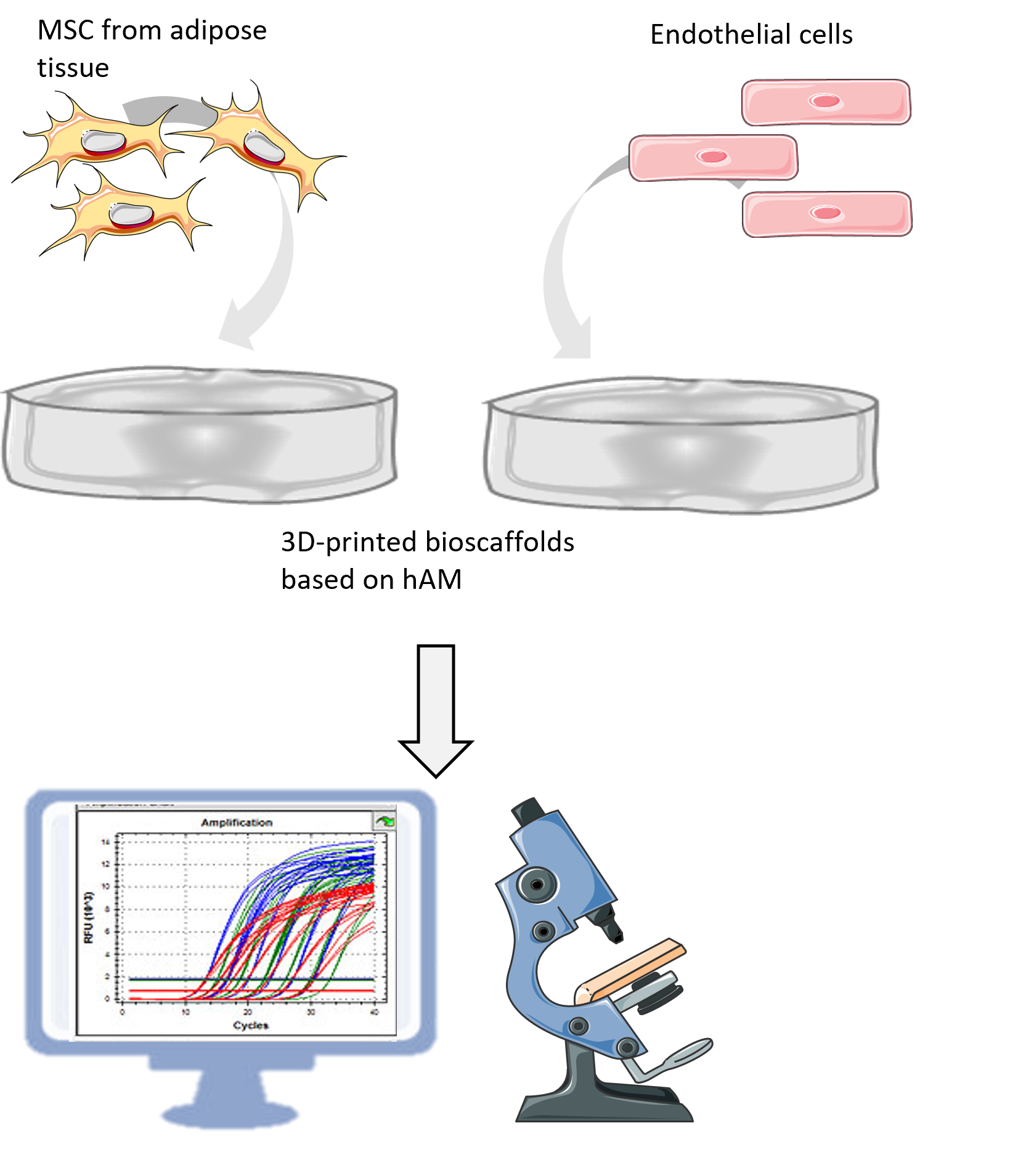
WORK PACKAGE 2
Breast tissue is a vascularized tissue consisting of glandular (breast lobes and ducts), fibrous (supportive or connective tissue) and interstitial fat tissue. Adipose tissue is the main component of the breast tissue niche where, in addition to adipocytes (fat tissue cells), there are cells of the stromal-vascular fraction, which includes mesenchymal stem cells (AT-MSC), endothelial cells, myoepithelial cells and immune cells. Within this work package, we will examine whether 3D printed biocarriers from hAM homogenate can support breast tissue reconstruction primarily in terms of adipose tissue regeneration, and we will test biocompatibility on AT-MSC and endothelial cell lines in vitro.
WORK PACKAGE 3
Although breast tumor removal is a successful life-saving operation, it still cannot ensure the elimination of cancer recurrence. The chances of recurrence of the disease increase with the reduction of the radicalization of the mastectomy. Although reconstructive surgery improves the patient's quality of life, there is still a risk of potentially retained "dormant" cancer cells. With that in mind, the second goal of this project is to investigate the antitumor effects of 3D printed biocarriers in vitro using different non-invasive and invasive breast tumor cell lines. In this sense, cell adhesion and migration of cancer cells to bioscaffolds will be examined, as well as the impact on survival, multiplication, expression of CSC (cancer stem cell) phenotype of aggressive breast cancer cell lines.
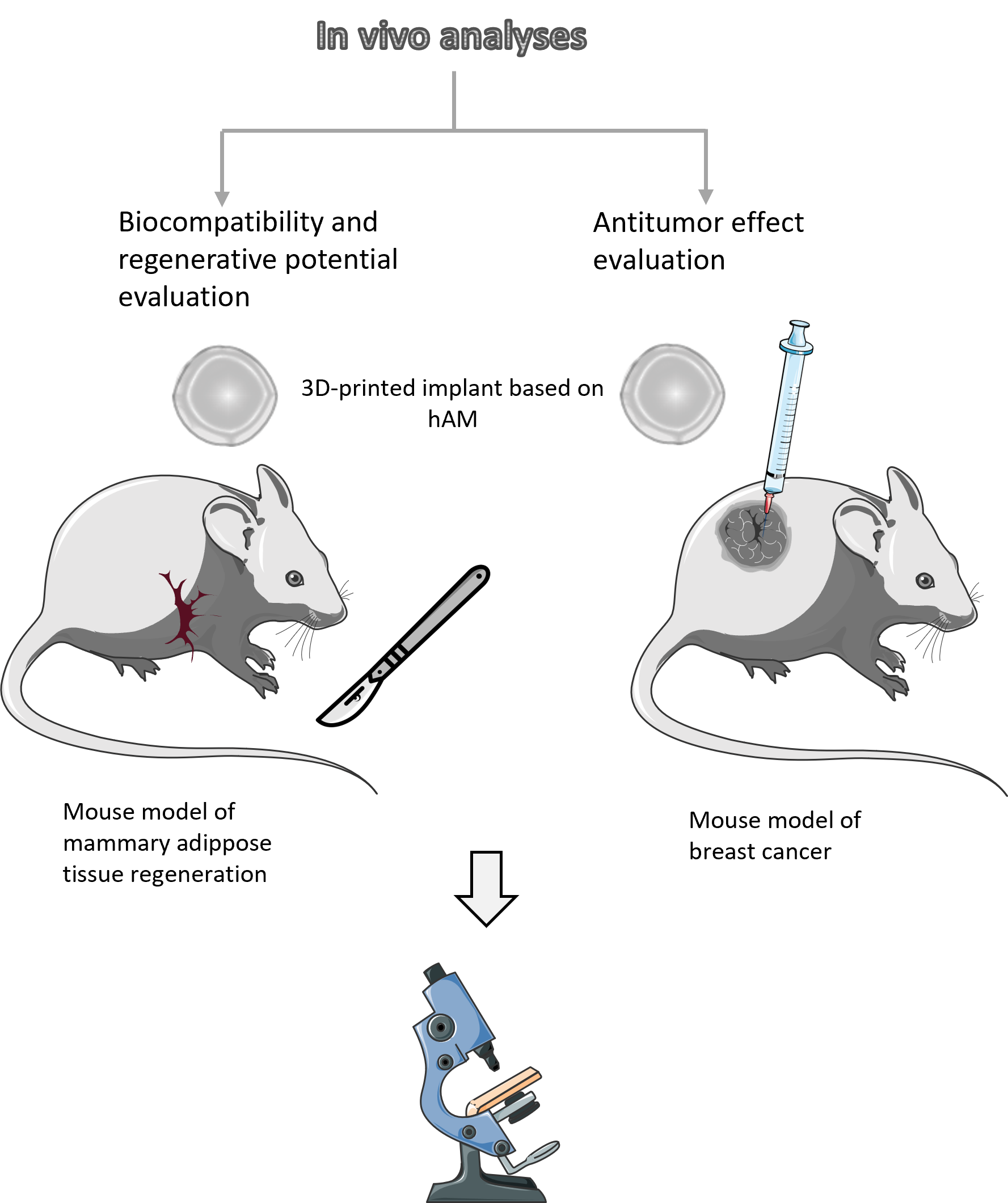
WORK PACKAGE 4
In order to examine and confirm the effects of 3D printed biocarriers in the physiological environment of the mammalian organism, in vivo studies will be conducted on mice. This research will be divided into two experimental settings. One will aim to test the biocompatibility and regenerative potential of a 3D printed implant based on hAM homogenate after implantation in a healthy animal that underwent a radical mastectomy. Tissue regeneration and vascularization will be monitored by histological observation. Another experimental setup will test the anti-tumor effect of the implant, by implanting a 3D-printed implant near the site of the tumor in mice with breast tumors. After a predetermined period of time, specific analyzes such as tumor size measurement and histopathology will be performed.